Colour Mixing – a guide for contemporary artists
The artists’ palette of colours is a rainbow, but unlike its sky-borne counterpart, our colours are constructed from pigments, fine grains of colour that must be bound together in order to become paint. The physical nature of pigments often interferes with the purity of perceived colour, but there has been available for more than fifty years the option of ‘modern’ colours allowing us to replicate the vibrancy of light itself.
To begin with, what exactly is colour? We perceive colour when white light strikes a surface, certain parts of the full light spectrum are absorbed, that which is not, is reflected back to our eye. So the red visible to our eye is a pigment absorbing most of the blue and yellow wavelength of light that strikes it, only the red spectrum is bounced back for us to ‘see’.
Pigments can be naturally occurring (such as the ochres) or man-made (such as ultramarines and dioxazines). They can be made from minerals, plants and complex petroleum derivatives.
To explain the dramatic differences between traditional and modern colours we will define, for the purpose of this article, colours by the chemical composition of the pigments:
Traditional pigments are inorganic in construction and are mostly based on so-called transition metals such as lead, iron and cadmium. Modern pigments are organic, constructed by rearranging carbon and oxygen molecules to create new compounds to yield colours of even greater brilliance. The complex chemical nature of these pigments results in the similarly complex names of the colours such as Phthalocyanine, Diarylide and Quinacridone.
We are not saying that artists should abandon traditional pigments, far from it. Colours such as the Ochres, Cobalts and Cadmiums have rightfully secured a permanent place in our palette. What we hope to illustrate is that colours available to the artist today are beyond the imagination of previous generations and their inclusion will create solutions to many artists’ problems. And for artists wanting to use a limited number of paints but who want to create a vast spectrum of colour the modern pigments open up dazzling opportunities.
Traditional and Modern Colour Wheels
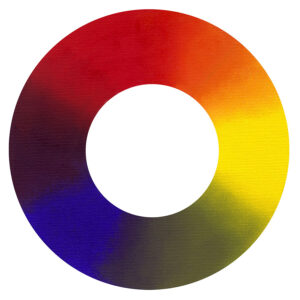
Traditional Colour Wheel
Cadmium Yellow/Cadmium Red/Cobalt Blue

Modern Colour Wheel
Arylide Yellow/Quinacridone Magenta/Phthalo Blue
Above are two colour wheels, one from traditional, the other with modern pigments. Note how the modern colours, especially the blends from the primaries are extremely clean and bright. Because the traditional primaries are lower in chromatic value (saturation of colour), blending the two often creates dull secondaries and very dull tertiaries. Although the modern primary of magenta is not a colour most would use straight from the tube, its extraordinary ability to make vibrant violets and red-orange blends explains its importance. Similarly, Phthalo Blue can be an awkward colour in itself, often so bitter when used undiluted but will mix the cleanest of greens and violets.
Opaque vs Transparent
Traditional colours are generally more opaque, that is they block out colour underneath.
This is particularly useful in ‘direct’ painting, applying strokes of colour over each other for bold mark making. Modern ‘organic’ pigments are, due to the fineness of the pigment particle, much more transparent and do not block out the image underneath as successfully. Many artists have struggled trying to make opaque films of paint with transparent colours. Do not fight their nature, but know that modern organic pigments are not weak, just naturally transparent.
Conversely, if wanting to make opaque colours transparent they have to be diluted dramatically and so lose colour strength. Select pigments that work with you, not against you.
Arylide Yellow

Quinacridone Magenta

Phthalocyanine Blue (G/S)

Cadmium Yellow

Cadmium Red

Cobalt Blue
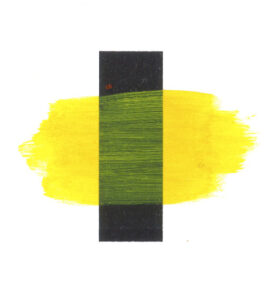
Naturally occurring pigments such as ochres have been used since prehistoric times. Humans have been resourceful and inventive and we have discovered many ways of making imagined colour real. The history of making colours not found in nature goes back to the ancient Egyptians but even with our inventiveness the range of colour available before the Industrial Revolution was technically limited. Most of the pigments in use were earth and mineral pigments, or pigments of biological origin. Pigments came from unusual sources such as plants, insects or naturally occurring combinations of chemicals and many were costly or impossible to intermix. Artists had to be inventive in creating bright colours from pigments available to them and discovered different methods to achieve these colours. Nature is not abundant in pigment greens, so most had to made by using blue and yellow but this came at a cost. Even the strongest available pigments when mixed together start to ‘grey-out’ and their brilliance is lost. Below are three examples of achieving a green. The first is a mixture of Cadmium Yellow and Cobalt Blue but the resulting green has lost the intensity one would expect. The second example uses the same two pigments but by altering the colour application has maintained purer colour. By laying a transparent glaze of yellow over the blue the resulting green is more brilliant. Glazing was a necessity to artists historically because of the limitations of pigment technology. The use of glazing was introduced for multiple reasons. Optically it allowed artists to describe rich colour, as not only does the layered colour stay cleaner, but light passing through the transparent films shines from within as it bounces back from the colour underneath. Secondly, many pigments, before modern chemistry, were highly reactive and corrupted when in direct contact with each other. Finally, and probably a proscription built from experiencing the corruption of mixed colours just described, artists were instructed not to mix colours together.
The final example demonstrates the clarity of secondary mixes using modern organic pigments. No loss of chroma, very pure and brilliant.

Green mix: Cobalt Blue and Cadmium Yellow

Glazing Cadmium Yellow over Cobalt Blue

Green mix: Phthalo Blue and Arylide Yellow
One more thing on the use of blended colours; In colour science it is well known that when a secondary colour (orange, violet, green) can be made as a single pigment it will more likely be chromatically stronger than two primaries mixed together.
Below left is a swatch of Cadmium Orange pigment. It is not a blend, but a single pigment. Next to it is a Cadmium Orange made by blending Cadmiums Red and Yellow. It is made from two distinct pigments. It cannot compete with its single pigment cousin. The final example below is a mixture of modern pigments that illustrates the ease with which these colours can mix and still create vibrant orange.
However, even with modern pigments the advantages of single pigment over mixes holds true. A Phthalo Green pigment is more chromatically intense than a green made with Phthalo Blue and Arylide Yellow. As more pigments are introduced, more of the colour is knocked off the reflected light, ending with loss of purity.

Single pigment: Cadmium Orange

Orange mix: Cadmium Red and Cadmium Yellow

Orange mix: Quin Magenta and Arylide Yellow
Modern organic pigments are inventions of the early twentieth century, but many have taken a long time to be accepted within the artists’ palette. Our conservatism is due in part because a full explanation of their capabilities is only now becoming apparent.
The colours available now are immense, far greater that at any time in human history. We are not advocating the purchase of every colour manufactured, but that building a ‘tool-box’ of colours to perform specific tasks gives us freedom to weave the rainbow beyond the bounds of earlier imaginations.




